Water Quality Monitoring Sites and Objectives
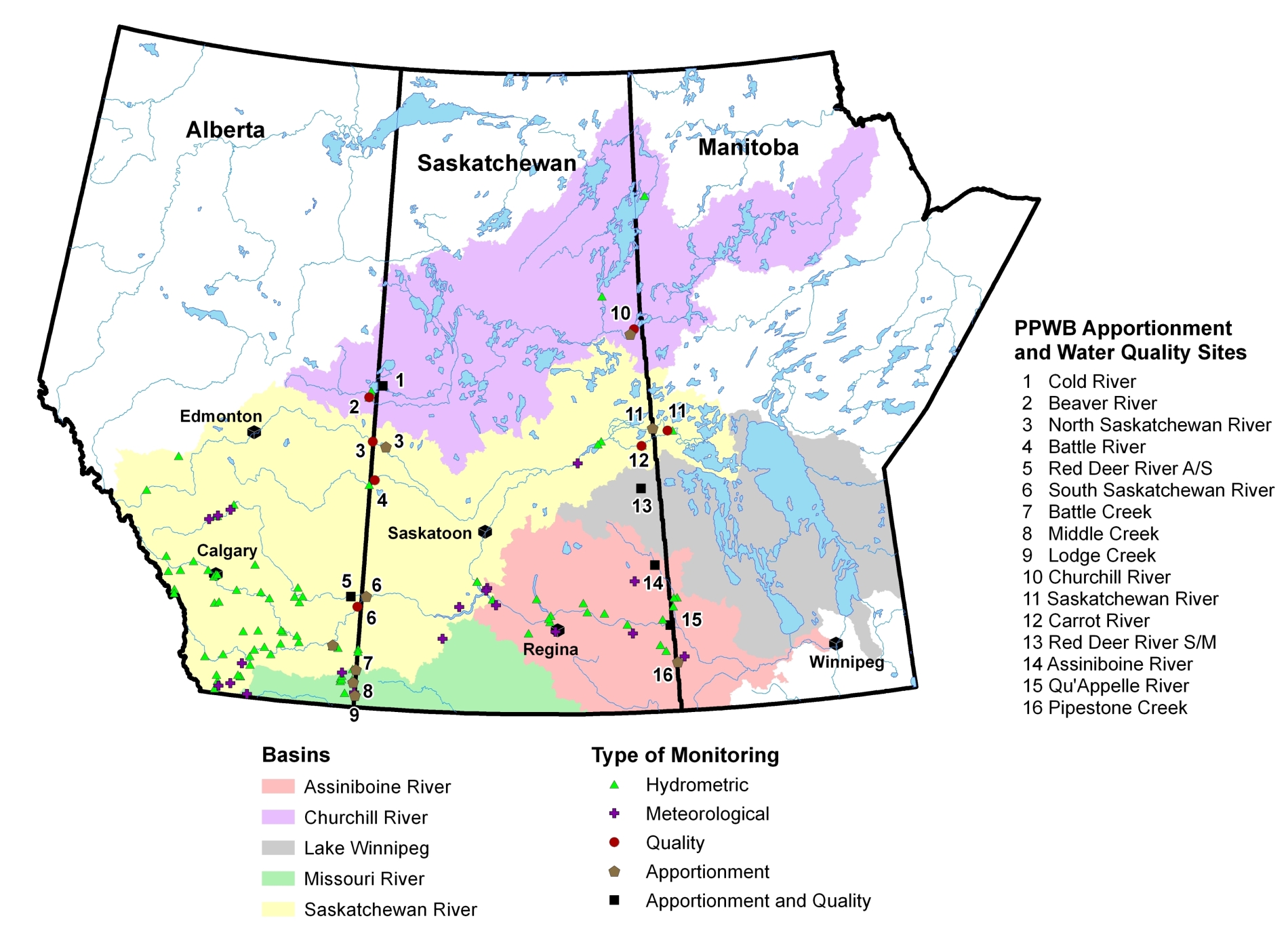
Schedule E of the Master Agreement on Apportionment contains PPWB water quality objectives for 12 interprovincial river reaches. Environment and Climate Change Canada monitors water quality at these 12 sites for the PPWB. The complete list of water quality objectives for the 12 sites is available here and is also available below for each river.
Total Ammonia Nitrogen (mg/L) **
The toxicity of ammonia relates primarily to the un-ionized form (NH3). The concentration of un-ionized ammonia present in water increases with pH and temperature. The values below represent total ammonia-nitrogen concentrations (at various temperatures and pH levels) above which accompanying NH3 concentrations may be harmful to aquatic life.
Total Ammonia (NH3 + NH4+)
(maximum levels expressed as N at various pH/temperature conditions)
Toxicity of Ammonia under varying Temperature and pH Conditions | |||||||
| 0° | 5° | 10° | 15° | 20° | 25° | 30° |
6.50 | 2.06 | 1.97 | 1.81 | 1.81 | 1.22 | 0.85 | 0.60 |
6.75 | 2.06 | 1.97 | 1.81 | 1.81 | 1.22 | 0.85 | 0.61 |
7.00 | 2.06 | 1.97 | 1.81 | 1.81 | 1.22 | 0.85 | 0.61 |
7.25 | 2.06 | 1.97 | 1.81 | 1.81 | 1.23 | 0.86 | 0.61 |
7.50 | 2.06 | 1.97 | 1.81 | 1.81 | 1.23 | 0.87 | 0.62 |
7.75 | 1.89 | 1.81 | 1.73 | 1.64 | 1.15 | 0.81 | 0.58 |
8.00 | 1.26 | 1.18 | 1.13 | 1.09 | 0.76 | 0.54 | 0.39 |
8.25 | 0.72 | 0.67 | 0.64 | 0.62 | 0.44 | 0.32 | 0.23 |
8.50 | 0.40 | 0.39 | 0.37 | 0.37 | 0.26 | 0.19 | 0.15 |
8.75 | 0.23 | 0.22 | 0.21 | 0.22 | 0.16 | 0.12 | 0.09 |
9.00 | 0.13 | 0.13 | 0.13 | 0.13 | 0.11 | 0.08 | 0.06 |
** excerpt from the "Surface Water Quality Objectives", Water Quality Branch Saskatchewan Environment and Public Safety, November, 1988 (WQ 110)
Cold River Reach: Outlet of Cold Lake | |||
Chemical, Physical or Biological Variable | Unit | Acceptable Limit or Limits | |
Nutrients | Open | Closed | |
Total Phosphorus | mg/L | 0.023 | 0.024 |
Total Dissolved Phosphorus | mg/L | 0.010 | 0.017 |
Total Nitrogen | mg/L | 0.453 | 0.452 |
0.460 | 0.467 | ||
Nitrate as N | mg/L | 3 | |
Ammonia Un-ionized | mg/L | 0.019a | |
Major Ions |
|
| |
Total Dissolved Solids | mg/L | 500 | |
Sulphate Dissolved | mg/L | 250 | |
Sodium Dissolved | mg/L | 200 | |
Fluoride Dissolved | mg/L | 0.12 | |
Chloride Dissolved | mg/L | 100 | |
Physicals and Other |
| ||
pH Lab | pH units | 6.5-9.0 | |
pH Field | pH units | 6.5-9.0 | |
Oxygen Dissolved |
|
| |
Temperature > 5°C (Open Season) | mg/L | 5 | |
Temperature < 5°C (Closed Season) | mg/L | 3 | |
Sodium Adsorption Ratio | rel units | 3 | |
Total Suspended Solids | mg/L | 1.2-4.8 | |
Reactive Chlorine Species | mg/L | 0.0005 | |
Cyanide (free) | mg/L | 0.005 | |
|
| ||
E. Coli | No./100 mL | 200 | |
Coliforms Fecal | No./100 mL | 100 | |
Metals |
| ||
Arsenic Total | µg/L | 5 | |
Arsenic Dissolved | µg/L | No Objective | |
Barium Total | µg/L | 1000 | |
Beryllium Total | µg/L | 100 | |
Boron Total | µg/L | 500 | |
Cadmium Total | µg/L | Calculatedb | |
Chromium Total | µg/L | 50 | |
Cobalt Total | µg/L | 50 | |
Copper Total | µg/L | Calculatedb | |
Iron Dissolved | µg/L | 300 | |
Lead Total | µg/L | Calculatedb | |
Lithium Total | µg/L | 2500 | |
Manganese Dissolved | µg/L | 50 | |
Mercury Total | µg/L | 0.026 | |
Molybdenum Total | µg/L | 10 | |
Nickel Dissolved | µg/L | Calculatedb | |
Selenium Total | µg/L | 1 | |
Silver Total | µg/L | 0.1 | |
Thallium Total | µg/L | 0.8 | |
Uranium Total | µg/L | 10 | |
Vanadium Total | µg/L | 100 | |
Zinc Total | µg/L | 30 | |
Pesticides |
|
|
Acid Herbicides |
|
|
2,4-D | µg/L | 4 |
Bromoxynil | µg/L | 0.33 |
Dicamba | µg/L | 0.006 |
MCPA | µg/L | 0.025 |
Picloram | µg/L | 29 |
Organochlorine Pesticides in Water |
|
|
Endosulfan | µg/L | 0.003 |
Hexachlorocyclohexane (gamma-HCH) (Lindane) | µg/L | 0.01 |
Hexachlorobenzene | µg/L | 0.52 |
Pentachlorophenol (PCP) | µg/L | 0.5 |
Neutral Herbicides in Water |
|
|
Atrazine | µg/L | 1.8 |
Diclofopmethyl (Hoegrass) | µg/L | 0.18 |
Metolachlor | µg/L | 7.8 |
Metribuzin | µg/L | 0.5 |
Simazine | µg/L | 0.5 |
Triallate | µg/L | 0.24 |
Trifluralin | µg/L | 0.2 |
Other |
|
|
Glyphosate | µg/L | Report Detections |
Fish Tissue |
|
|
Mercury in fish (muscle tissue) | µg/kg | 200 |
Arsenic in fish (muscle tissue) | µg/kg | 3500 |
Lead in fish (muscle tissue) | µg/kg | 500 |
DDT (total) in fish (muscle tissue) | µg/kg | 5000 |
Aquatic Biota Consumption |
|
|
PCB in fish (muscle tissue) mammalian | µg TEQ/kg diet wet weight | 0.00079 |
PCB in fish (muscle tissue) avian | µg TEQ/kg diet wet weight | 0.0024 |
DDT (total) in fish (muscle tissue) | µg/kg diet wet weight | 14 |
Toxaphene in fish (muscle tissue) | µg/kg diet wet weight | 6.3 |
Radioactive |
|
|
Cesium-137 | Bq/L | 10 |
Iodine-131 | Bq/L | 6 |
Lead-210 | Bq/L | 0.2 |
Radium-226 | Bq/L | 0.5 |
Strontium-90 | Bq/L | 5 |
Tritium | Bq/L | 7000 |
Assiniboine River Reach: Whitesand River to Outlet of Shellmouth Reservoir | |||
Chemical, Physical or Biological Variable | Unit | Acceptable Limit or Limits | |
Nutrients | Open | Closed | |
Total Phosphorus | mg/L | 0.311 | 0.180 |
Total Dissolved Phosphorus | mg/L | 0.186 | 0.115 |
Total Nitrogen | mg/L | 1.801 | 2.252 |
Nitrate as N | mg/L | 3 | |
Ammonia Un-ionized | mg/L | 0.019a | |
Major Ions |
|
| |
Total Dissolved Solids | mg/L | 834 | |
Sulphate Dissolved | mg/L | 299 | |
Sodium Dissolved | mg/L | 200 | |
Fluoride Dissolved | mg/L | 0.26 | |
Chloride Dissolved | mg/L | 100 | |
Physicals and Other | |||
pH Lab | pH units | 6.5-9.0 | |
pH Field | pH units | 6.5-9.0 | |
Oxygen Dissolved |
|
| |
Temperature > 5°C (Open Season) | mg/L | 5 | |
Temperature < 5°C (Closed Season) | mg/L | 3 | |
Sodium Adsorption Ratio | rel units | 3 | |
Total Suspended Solids | mg/L | 5.0-69.2 | |
Reactive Chlorine Species | mg/L | 0.0005 | |
Cyanide (free) | mg/L | 0.005 | |
Biota | |||
E. Coli | No./100 mL | 200 | |
Coliforms Fecal | No./100 mL | 100 | |
Metals | |||
Arsenic Total | µg/L | 5 | |
Arsenic Dissolved | µg/L | No Objective | |
Barium Total | µg/L | 1000 | |
Beryllium Total | µg/L | 100 | |
Boron Total | µg/L | 500 | |
Cadmium Total | µg/L | Calculatedb | |
Chromium Total | µg/L | 50 | |
Cobalt Total | µg/L | 50 | |
Copper Total | µg/L | Calculatedb | |
Iron Dissolved | µg/L | 300 | |
Lead Total | µg/L | Calculatedb | |
Lithium Total | µg/L | 2500 | |
Manganese Dissolved | µg/L | Under Review | |
Mercury Total | µg/L | 0.026 | |
Molybdenum Total | µg/L | 10 | |
Nickel Dissolved | µg/L | Calculatedb | |
Selenium Total | µg/L | 1 | |
Silver Total | µg/L | 0.1 | |
Thallium Total | µg/L | 0.8 | |
Uranium Total | µg/L | 10 | |
Vanadium Total | µg/L | 100 | |
Zinc Total | µg/L | 30 | |
Pesticides |
|
|
Acid Herbicides |
|
|
2,4-D | µg/L | 4 |
Bromoxynil | µg/L | 0.33 |
Dicamba | µg/L | 0.006 |
MCPA | µg/L | 0.025 |
Picloram | µg/L | 29 |
Organochlorine Pesticides in Water |
|
|
Endosulfan | µg/L | 0.003 |
Hexachlorocyclohexane (gamma-HCH) (Lindane) | µg/L | 0.01 |
Hexachlorobenzene | µg/L | 0.52 |
Pentachlorophenol (PCP) | µg/L | 0.5 |
Neutral Herbicides in Water |
|
|
Atrazine | µg/L | 1.8 |
Diclofopmethyl (Hoegrass) | µg/L | 0.18 |
Metolachlor | µg/L | 7.8 |
Metribuzin | µg/L | 0.5 |
Simazine | µg/L | 0.5 |
Triallate | µg/L | 0.24 |
Trifluralin | µg/L | 0.2 |
Other |
|
|
Glyphosate | µg/L | Report Detections |
Fish Tissue |
|
|
Mercury in fish (muscle tissue) | µg/kg | 200 |
Arsenic in fish (muscle tissue) | µg/kg | 3500 |
Lead in fish (muscle tissue) | µg/kg | 500 |
DDT (total) in fish (muscle tissue) | µg/kg | 5000 |
Aquatic Biota Consumption |
|
|
PCB in fish (muscle tissue) mammalian | µg TEQ/kg diet wet weight | 0.00079 |
PCB in fish (muscle tissue) avian | µg TEQ/kg diet wet weight | 0.0024 |
DDT (total) in fish (muscle tissue) | µg/kg diet wet weight | 14 |
Toxaphene in fish (muscle tissue) | µg/kg diet wet weight | 6.3 |
Radioactive |
|
|
Cesium-137 | Bq/L | 10 |
Iodine-131 | Bq/L | 6 |
Lead-210 | Bq/L | 0.2 |
Radium-226 | Bq/L | 0.5 |
Strontium-90 | Bq/L | 5 |
Tritium | Bq/L | 7000 |
Battle River Reach: Blackfoot Creek to Unwin | |||
Chemical, Physical or Biological Variable | Unit | Acceptable Limit or Limits | |
Nutrients | Open | Closed | |
Total Phosphorus | mg/L | 0.267 | 0.075 |
0.335 | 0.100 | ||
Total Dissolved Phosphorus | mg/L | 0.051 | 0.045 |
Total Nitrogen | mg/L | 2.260 | 1.550 |
Nitrate as N | mg/L | 3 | |
Ammonia Un-ionized | mg/L | 0.019a | |
Major Ions |
|
| |
Total Dissolved Solids | mg/L | 872 | |
Sulphate Dissolved | mg/L | 250 | |
Sodium Dissolved | mg/L | 200 | |
Fluoride Dissolved | mg/L | 0.31 | |
Chloride Dissolved | mg/L | 100 | |
Physicals and Other | |||
pH Lab | pH units | 6.5-9.0 | |
pH Field | pH units | 6.5-9.0 | |
Oxygen Dissolved |
|
| |
Temperature > 5°C (Open Season) | mg/L | 5 | |
Temperature < 5°C (Closed Season) | mg/L | Under Review | |
Sodium Adsorption Ratio | rel units | Under Review | |
Total Suspended Solids | mg/L | 5.0 - 320.0 | |
Reactive Chlorine Species | mg/L | 0.0005 | |
Cyanide (free) | mg/L | 0.005 | |
Biota | |||
E. Coli | No./100 mL | 200 | |
Coliforms Fecal | No./100 mL | 100 | |
Metals | |||
Arsenic Total | µg/L | 5 | |
Arsenic Dissolved | µg/L | No Objective | |
Barium Total | µg/L | 1000 | |
Beryllium Total | µg/L | 100 | |
Boron Total | µg/L | 500 | |
Cadmium Total | µg/L | Calculatedb | |
Chromium Total | µg/L | 50 | |
Cobalt Total | µg/L | 50 | |
Copper Total | µg/L | Calculatedb | |
Iron Dissolved | µg/L | 300 | |
Lead Total | µg/L | Calculatedb | |
Lithium Total | µg/L | 2500 | |
Manganese Dissolved | µg/L | Under Review | |
Mercury Total | µg/L | 0.026 | |
Molybdenum Total | µg/L | 10 | |
Nickel Dissolved | µg/L | Calculatedb | |
Selenium Total | µg/L | 1 | |
Silver Total | µg/L | 0.1 | |
Thallium Total | µg/L | 0.8 | |
Uranium Total | µg/L | 10 | |
Vanadium Total | µg/L | 100 | |
Zinc Total | µg/L | 30 | |
Pesticides |
|
|
Acid Herbicides |
|
|
2,4-D | µg/L | 4 |
Bromoxynil | µg/L | 0.33 |
Dicamba | µg/L | 0.006 |
MCPA | µg/L | 0.025 |
Picloram | µg/L | 29 |
Organochlorine Pesticides in Water |
|
|
Endosulfan | µg/L | 0.003 |
Hexachlorocyclohexane (gamma-HCH) (Lindane) | µg/L | 0.01 |
Hexachlorobenzene | µg/L | 0.52 |
Pentachlorophenol (PCP) | µg/L | 0.5 |
Neutral Herbicides in Water |
|
|
Atrazine | µg/L | 1.8 |
Diclofopmethyl (Hoegrass) | µg/L | 0.18 |
Metolachlor | µg/L | 7.8 |
Metribuzin | µg/L | 0.5 |
Simazine | µg/L | 0.5 |
Triallate | µg/L | 0.24 |
Trifluralin | µg/L | 0.2 |
Other |
|
|
Glyphosate | µg/L | Report Detections |
Fish Tissue |
|
|
Mercury in fish (muscle tissue) | µg/kg | 200 |
Arsenic in fish (muscle tissue) | µg/kg | 3500 |
Lead in fish (muscle tissue) | µg/kg | 500 |
DDT (total) in fish (muscle tissue) | µg/kg | 5000 |
Aquatic Biota Consumption |
|
|
PCB in fish (muscle tissue) mammalian | µg TEQ/kg diet wet weight | 0.00079 |
PCB in fish (muscle tissue) avian | µg TEQ/kg diet wet weight | 0.0024 |
DDT (total) in fish (muscle tissue) | µg/kg diet wet weight | 14 |
Toxaphene in fish (muscle tissue) | µg/kg diet wet weight | 6.3 |
Radioactive |
|
|
Cesium-137 | Bq/L | 10 |
Iodine-131 | Bq/L | 6 |
Lead-210 | Bq/L | 0.2 |
Radium-226 | Bq/L | 0.5 |
Strontium-90 | Bq/L | 5 |
Tritium | Bq/L | 7000 |
Beaver River Reach: Beaver Crossing to the Border | |||
Chemical, Physical or Biological Variable | Unit | Acceptable Limit or Limits | |
Nutrients | Open | Closed | |
Total Phosphorus | mg/L | 0.171 | 0.127 |
Total Dissolved Phosphorus | mg/L | 0.043 | 0.042 |
0.060 | 0.060 | ||
Total Nitrogen | mg/L | 1.140 | 1.862 |
Nitrate as N | mg/L | 3 | |
Ammonia Un-ionized | mg/L | 0.019a | |
Major Ions |
|
| |
Total Dissolved Solids | mg/L | 500 | |
Sulphate Dissolved | mg/L | 250 | |
Sodium Dissolved | mg/L | 200 | |
Fluoride Dissolved | mg/L | 0.19 | |
Chloride Dissolved | mg/L | 100 | |
Physicals and Other | |||
pH Lab | pH units | 6.5-9.0 | |
pH Field | pH units | 6.5-9.0 | |
Oxygen Dissolved |
|
| |
Temperature > 5°C (Open Season) | mg/L | 5 | |
Temperature < 5°C (Closed Season) | mg/L | Under Review | |
Sodium Adsorption Ratio | rel units | 3 | |
Total Suspended Solids | mg/L | 3.0-48.8 | |
Reactive Chlorine Species | mg/L | 0.0005 | |
Cyanide (free) | mg/L | 0.005 | |
| |||
E. Coli | No./100 mL | 200 | |
Coliforms Fecal | No./100 mL | 100 | |
Metals | |||
Arsenic Total | µg/L | 5 | |
Arsenic Dissolved | µg/L | No Objective | |
Barium Total | µg/L | 1000 | |
Beryllium Total | µg/L | 100 | |
Boron Total | µg/L | 500 | |
Cadmium Total | µg/L | Calculatedb | |
Chromium Total | µg/L | 50 | |
Cobalt Total | µg/L | 50 | |
Copper Total | µg/L | Calculatedb | |
Iron Dissolved | µg/L | 300 | |
Lead Total | µg/L | Calculatedb | |
Lithium Total | µg/L | 2500 | |
Manganese Dissolved | µg/L | Under Review | |
Mercury Total | µg/L | 0.026 | |
Molybdenum Total | µg/L | 10 | |
Nickel Dissolved | µg/L | Calculatedb | |
Selenium Total | µg/L | 1 | |
Silver Total | µg/L | 0.1 | |
Thallium Total | µg/L | 0.8 | |
Uranium Total | µg/L | 10 | |
Vanadium Total | µg/L | 100 | |
Zinc Total | µg/L | 30 | |
Pesticides |
|
|
Acid Herbicides |
|
|
2,4-D | µg/L | 4 |
Bromoxynil | µg/L | 0.33 |
Dicamba | µg/L | 0.006 |
MCPA | µg/L | 0.025 |
Picloram | µg/L | 29 |
Organochlorine Pesticides in Water |
|
|
Endosulfan | µg/L | 0.003 |
Hexachlorocyclohexane (gamma-HCH) (Lindane) | µg/L | 0.01 |
Hexachlorobenzene | µg/L | 0.52 |
Pentachlorophenol (PCP) | µg/L | 0.5 |
Neutral Herbicides in Water |
|
|
Atrazine | µg/L | 1.8 |
Diclofopmethyl (Hoegrass) | µg/L | 0.18 |
Metolachlor | µg/L | 7.8 |
Metribuzin | µg/L | 0.5 |
Simazine | µg/L | 0.5 |
Triallate | µg/L | 0.24 |
Trifluralin | µg/L | 0.2 |
Other |
|
|
Glyphosate | µg/L | Report Detections |
Fish Tissue |
|
|
Mercury in fish (muscle tissue) | µg/kg | 200 |
Arsenic in fish (muscle tissue) | µg/kg | 3500 |
Lead in fish (muscle tissue) | µg/kg | 500 |
DDT (total) in fish (muscle tissue) | µg/kg | 5000 |
Aquatic Biota Consumption |
|
|
PCB in fish (muscle tissue) mammalian | µg TEQ/kg diet wet weight | 0.00079 |
PCB in fish (muscle tissue) avian | µg TEQ/kg diet wet weight | 0.0024 |
DDT (total) in fish (muscle tissue) | µg/kg diet wet weight | 14 |
Toxaphene in fish (muscle tissue) | µg/kg diet wet weight | 6.3 |
Radioactive |
|
|
Cesium-137 | Bq/L | 10 |
Iodine-131 | Bq/L | 6 |
Lead-210 | Bq/L | 0.2 |
Radium-226 | Bq/L | 0.5 |
Strontium-90 | Bq/L | 5 |
Tritium | Bq/L | 7000 |
Carrot River Reach: Turnberry to Mouth of Carrot River | |||
Chemical, Physical or Biological Variable | Unit | Acceptable Limit or Limits | |
Nutrients | Open | Closed | |
Total Phosphorus | mg/L | 0.099 | 0.170 |
0.140 | 0.266 | ||
Total Dissolved Phosphorus | mg/L | 0.027 | 0.031 |
0.057 | 0.059 | ||
Total Nitrogen | mg/L | 1.087 | 1.814 |
1.417 | 2.052 | ||
Nitrate as N | mg/L | 3 | |
Ammonia Un-ionized | mg/L | 0.019a | |
Major Ions |
|
| |
Total Dissolved Solids | mg/L | 742 | 1672 |
Sulphate Dissolved | mg/L | 250 | |
Sodium Dissolved | mg/L | 164 | 442 |
Fluoride Dissolved | mg/L | 0.2 | 0.29 |
Chloride Dissolved | mg/L | 267 | 728 |
Physicals and Other | |||
pH Lab | pH units | 6.5-9.0 | |
pH Field | pH units | 6.5-9.0 | |
Oxygen Dissolved |
|
| |
Temperature > 5°C (Open Season) | mg/L | 5 | |
Temperature < 5°C (Closed Season) | mg/L | Under Review | |
Sodium Adsorption Ratio | rel units | Under Review | |
Total Suspended Solids | mg/L | 6.08 -98.2 | |
Reactive Chlorine Species | mg/L | 0.0005 | |
Cyanide (free) | mg/L | 0.005 | |
| |||
E. Coli | No./100 mL | 200 | |
Coliforms Fecal | No./100 mL | 100 | |
Metals | |||
Arsenic Total | µg/L | No Objective | |
Arsenic Dissolved | µg/L | 50 | |
Barium Total | µg/L | 1000 | |
Beryllium Total | µg/L | 100 | |
Boron Total | µg/L | 500 | |
Cadmium Total | µg/L | Calculatedb | |
Chromium Total | µg/L | 50 | |
Cobalt Total | µg/L | 50 | |
Copper Total | µg/L | Calculatedb | |
Iron Dissolved | µg/L | Under Review | |
Lead Total | µg/L | Calculatedb | |
Lithium Total | µg/L | 2500 | |
Manganese Dissolved | µg/L | Under Review | |
Mercury Total | µg/L | 0.026 | |
Molybdenum Total | µg/L | 10 | |
Nickel Dissolved | µg/L | Calculatedb | |
Selenium Total | µg/L | 1 | |
Silver Total | µg/L | 0.1 | |
Thallium Total | µg/L | 0.8 | |
Uranium Total | µg/L | 10 | |
Vanadium Total | µg/L | 100 | |
Zinc Total | µg/L | 30 | |
Pesticides |
|
|
Acid Herbicides |
|
|
2,4-D | µg/L | 4 |
Bromoxynil | µg/L | 0.33 |
Dicamba | µg/L | 0.006 |
MCPA | µg/L | 0.025 |
Picloram | µg/L | 29 |
Organochlorine Pesticides in Water |
|
|
Endosulfan | µg/L | 0.003 |
Hexachlorocyclohexane (gamma-HCH) (Lindane) | µg/L | 0.01 |
Hexachlorobenzene | µg/L | 0.52 |
Pentachlorophenol (PCP) | µg/L | 0.5 |
Neutral Herbicides in Water |
|
|
Atrazine | µg/L | 1.8 |
Diclofopmethyl (Hoegrass) | µg/L | 0.18 |
Metolachlor | µg/L | 7.8 |
Metribuzin | µg/L | 0.5 |
Simazine | µg/L | 0.5 |
Triallate | µg/L | 0.24 |
Trifluralin | µg/L | 0.2 |
Other |
|
|
Glyphosate | µg/L | Report Detections |
Fish Tissue |
|
|
Mercury in fish (muscle tissue) | µg/kg | 200 |
Arsenic in fish (muscle tissue) | µg/kg | 3500 |
Lead in fish (muscle tissue) | µg/kg | 500 |
DDT (total) in fish (muscle tissue) | µg/kg | 5000 |
Aquatic Biota Consumption |
|
|
PCB in fish (muscle tissue) mammalian | µg TEQ/kg diet wet weight | 0.00079 |
PCB in fish (muscle tissue) avian | µg TEQ/kg diet wet weight | 0.0024 |
DDT (total) in fish (muscle tissue) | µg/kg diet wet weight | 14 |
Toxaphene in fish (muscle tissue) | µg/kg diet wet weight | 6.3 |
Radioactive |
|
|
Cesium-137 | Bq/L | 10 |
Iodine-131 | Bq/L | 6 |
Lead-210 | Bq/L | 0.2 |
Radium-226 | Bq/L | 0.5 |
Strontium-90 | Bq/L | 5 |
Tritium | Bq/L | 7000 |
Churchill River Reach: Island Falls to Pukatawagan Lake | |||
Chemical, Physical or Biological Variable | Unit | Acceptable Limit or Limits | |
Nutrients | Open | Closed | |
Total Phosphorus | mg/L | 0.025 | 0.021 |
Total Dissolved Phosphorus | mg/L | 0.010 | 0.010 |
Total Nitrogen | mg/L | 0.484 | 0.411 |
Nitrate as N | mg/L | 3 | |
Ammonia Un-ionized | mg/L | 0.019a | |
Major Ions |
|
| |
Total Dissolved Solids | mg/L | 500 | |
Sulphate Dissolved | mg/L | 250 | |
Sodium Dissolved | mg/L | 200 | |
Fluoride Dissolved | mg/L | 0.12 | |
Chloride Dissolved | mg/L | 100 | |
Physicals and Other | |||
pH Lab | pH units | 6.5-9.0 | |
pH Field | pH units | 6.5-9.0 | |
Oxygen Dissolved |
|
| |
Temperature > 5°C (Open Season) | mg/L | 5 | |
Temperature < 5°C (Closed Season) | mg/L | 3 | |
Sodium Adsorption Ratio | rel units | 3 | |
Total Suspended Solids | mg/L | 2.2-6.2 | |
Reactive Chlorine Species | mg/L | 0.0005 | |
Cyanide (free) | mg/L | 0.005 | |
Biota | |||
E. Coli | No./100 mL | 200 | |
Coliforms Fecal | No./100 mL | 100 | |
Metals | |||
Arsenic Total | µg/L | 5 | |
Arsenic Dissolved | µg/L | No Objective | |
Barium Total | µg/L | 1000 | |
Beryllium Total | µg/L | 100 | |
Boron Total | µg/L | 500 | |
Cadmium Total | µg/L | Calculatedb | |
Chromium Total | µg/L | 50 | |
Cobalt Total | µg/L | 50 | |
Copper Total | µg/L | Calculatedb | |
Iron Dissolved | µg/L | 300 | |
Lead Total | µg/L | Calculatedb | |
Lithium Total | µg/L | 2500 | |
Manganese Dissolved | µg/L | 50 | |
Mercury Total | µg/L | 0.026 | |
Molybdenum Total | µg/L | 10 | |
Nickel Dissolved | µg/L | Calculatedb | |
Selenium Total | µg/L | 1 | |
Silver Total | µg/L | 0.1 | |
Thallium Total | µg/L | 0.8 | |
Uranium Total | µg/L | 10 | |
Vanadium Total | µg/L | 100 | |
Zinc Total | µg/L | 30 | |
Pesticides |
|
|
Acid Herbicides |
|
|
2,4-D | µg/L | 4 |
Bromoxynil | µg/L | 0.33 |
Dicamba | µg/L | 0.006 |
MCPA | µg/L | 0.025 |
Picloram | µg/L | 29 |
Organochlorine Pesticides in Water |
|
|
Endosulfan | µg/L | 0.003 |
Hexachlorocyclohexane (gamma-HCH) (Lindane) | µg/L | 0.01 |
Hexachlorobenzene | µg/L | 0.52 |
Pentachlorophenol (PCP) | µg/L | 0.5 |
Neutral Herbicides in Water |
|
|
Atrazine | µg/L | 1.8 |
Diclofopmethyl (Hoegrass) | µg/L | 0.18 |
Metolachlor | µg/L | 7.8 |
Metribuzin | µg/L | 0.5 |
Simazine | µg/L | 0.5 |
Triallate | µg/L | 0.24 |
Trifluralin | µg/L | 0.2 |
Other |
|
|
Glyphosate | µg/L | Report Detections |
Fish Tissue |
|
|
Mercury in fish (muscle tissue) | µg/kg | 200 |
Arsenic in fish (muscle tissue) | µg/kg | 3500 |
Lead in fish (muscle tissue) | µg/kg | 500 |
DDT (total) in fish (muscle tissue) | µg/kg | 5000 |
Aquatic Biota Consumption |
|
|
PCB in fish (muscle tissue) mammalian | µg TEQ/kg diet wet weight | 0.00079 |
PCB in fish (muscle tissue) avian | µg TEQ/kg diet wet weight | 0.0024 |
DDT (total) in fish (muscle tissue) | µg/kg diet wet weight | 14 |
Toxaphene in fish (muscle tissue) | µg/kg diet wet weight | 6.3 |
Radioactive |
|
|
Cesium-137 | Bq/L | 10 |
Iodine-131 | Bq/L | 6 |
Lead-210 | Bq/L | 0.2 |
Radium-226 | Bq/L | 0.5 |
Strontium-90 | Bq/L | 5 |
Tritium | Bq/L | 7000 |
North Sask. River Reach: Lea Park to Lloydminster Ferry | |||
Chemical, Physical or Biological Variable | Unit | Acceptable Limit or Limits | |
Nutrients | Open | Closed | |
Total Phosphorus | mg/L | 0.253 | 0.063 |
0.278 | 0.115 | ||
Total Dissolved Phosphorus | mg/L | 0.026 | 0.048 |
0.046 | 0.101 | ||
Total Nitrogen | mg/L | 1.169 | 1.175 |
1.230 | 1.225 | ||
Nitrate as N | mg/L | 3 | |
Ammonia Un-ionized | mg/L | 0.019a | |
Major Ions |
|
| |
Total Dissolved Solids | mg/L | 500 | |
Sulphate Dissolved | mg/L | 250 | |
Sodium Dissolved | mg/L | 200 | |
Fluoride Dissolved | mg/L | 0.18 | |
Chloride Dissolved | mg/L | 100 | |
Physicals and Other | |||
pH Lab | pH units | 6.5-9.0 | |
pH Field | pH units | 6.5-9.0 | |
Oxygen Dissolved |
|
| |
Temperature > 5°C (Open Season) | mg/L | 5 | |
Temperature < 5°C (Closed Season) | mg/L | 3 | |
Sodium Adsorption Ratio | rel units | 3 | |
Total Suspended Solids | mg/L | 5.0-295.8 | |
Reactive Chlorine Species | mg/L | 0.0005 | |
Cyanide (free) | mg/L | 0.005 | |
| |||
E. Coli | No./100 mL | 200 | |
Coliforms Fecal | No./100 mL | 100 | |
Metals | |||
Arsenic Total | µg/L | 5 | |
Arsenic Dissolved | µg/L | No Objective | |
Barium Total | µg/L | 1000 | |
Beryllium Total | µg/L | 100 | |
Boron Total | µg/L | 500 | |
Cadmium Total | µg/L | Calculatedb | |
Chromium Total | µg/L | 50 | |
Cobalt Total | µg/L | 50 | |
Copper Total | µg/L | Calculatedb | |
Iron Dissolved | µg/L | 300 | |
Lead Total | µg/L | Calculatedb | |
Lithium Total | µg/L | 2500 | |
Manganese Dissolved | µg/L | 50 | |
Mercury Total | µg/L | 0.026 | |
Molybdenum Total | µg/L | 10 | |
Nickel Dissolved | µg/L | Calculatedb | |
Selenium Total | µg/L | 1 | |
Silver Total | µg/L | 0.1 | |
Thallium Total | µg/L | 0.8 | |
Uranium Total | µg/L | 10 | |
Vanadium Total | µg/L | 100 | |
Zinc Total | µg/L | 30 | |
Pesticides |
|
|
Acid Herbicides |
|
|
2,4-D | µg/L | 4 |
Bromoxynil | µg/L | 0.33 |
Dicamba | µg/L | 0.006 |
MCPA | µg/L | 0.025 |
Picloram | µg/L | 29 |
Organochlorine Pesticides in Water |
|
|
Endosulfan | µg/L | 0.003 |
Hexachlorocyclohexane (gamma-HCH) (Lindane) | µg/L | 0.01 |
Hexachlorobenzene | µg/L | 0.52 |
Pentachlorophenol (PCP) | µg/L | 0.5 |
Neutral Herbicides in Water |
|
|
Atrazine | µg/L | 1.8 |
Diclofopmethyl (Hoegrass) | µg/L | 0.18 |
Metolachlor | µg/L | 7.8 |
Metribuzin | µg/L | 0.5 |
Simazine | µg/L | 0.5 |
Triallate | µg/L | 0.24 |
Trifluralin | µg/L | 0.2 |
Other |
|
|
Glyphosate | µg/L | Report Detections |
Fish Tissue |
|
|
Mercury in fish (muscle tissue) | µg/kg | 200 |
Arsenic in fish (muscle tissue) | µg/kg | 3500 |
Lead in fish (muscle tissue) | µg/kg | 500 |
DDT (total) in fish (muscle tissue) | µg/kg | 5000 |
Aquatic Biota Consumption |
|
|
PCB in fish (muscle tissue) mammalian | µg TEQ/kg diet wet weight | 0.00079 |
PCB in fish (muscle tissue) avian | µg TEQ/kg diet wet weight | 0.0024 |
DDT (total) in fish (muscle tissue) | µg/kg diet wet weight | 14 |
Toxaphene in fish (muscle tissue) | µg/kg diet wet weight | 6.3 |
Radioactive |
|
|
Cesium-137 | Bq/L | 10 |
Iodine-131 | Bq/L | 6 |
Lead-210 | Bq/L | 0.2 |
Radium-226 | Bq/L | 0.5 |
Strontium-90 | Bq/L | 5 |
Tritium | Bq/L | 7000 |
Qu'Appelle River Reach: Kaposvar Creek to Assiniboine River | |||
Chemical, Physical or Biological Variable | Unit | Acceptable Limit or Limits | |
Nutrients | Open | Closed | |
Total Phosphorus | mg/L | 0.278 | 0.221 |
0.304 | 0.290 | ||
Total Dissolved Phosphorus | mg/L | 0.156 | 0.129 |
0.190 | 0.249 | ||
Total Nitrogen | mg/L | 1.822 | 1.767 |
Nitrate as N | mg/L | 3 | |
Ammonia Un-ionized | mg/L | 0.019a | |
Major Ions |
|
| |
Total Dissolved Solids | mg/L | 1144 | |
Sulphate Dissolved | mg/L | 486 | |
Sodium Dissolved | mg/L | 200 | |
Fluoride Dissolved | mg/L | 0.25 | |
Chloride Dissolved | mg/L | 100 | |
Physicals and Other | |||
pH Lab | pH units | 6.5-9.0 | |
pH Field | pH units | 6.5-9.0 | |
Oxygen Dissolved |
|
| |
Temperature > 5°C (Open Season) | mg/L | 5 | |
Temperature < 5°C (Closed Season) | mg/L | 3 | |
Sodium Adsorption Ratio | rel units | Under Review | |
Total Suspended Solids | mg/L | 22.6 -122.2 | |
Reactive Chlorine Species | mg/L | 0.0005 | |
Cyanide (free) | mg/L | 0.005 | |
| |||
E. Coli | No./100 mL | 200 | |
Coliforms Fecal | No./100 mL | 100 | |
Metals | |||
Arsenic Total | µg/L | No Objective | |
Arsenic Dissolved | µg/L | 50 | |
Barium Total | µg/L | 1000 | |
Beryllium Total | µg/L | 100 | |
Boron Total | µg/L | 500 | |
Cadmium Total | µg/L | Calculatedb | |
Chromium Total | µg/L | 50 | |
Cobalt Total | µg/L | 50 | |
Copper Total | µg/L | Calculatedb | |
Iron Dissolved | µg/L | 300 | |
Lead Total | µg/L | Calculatedb | |
Lithium Total | µg/L | 2500 | |
Manganese Dissolved | µg/L | Under Review | |
Mercury Total | µg/L | 0.026 | |
Molybdenum Total | µg/L | 10 | |
Nickel Dissolved | µg/L | Calculatedb | |
Selenium Total | µg/L | 1 | |
Silver Total | µg/L | 0.1 | |
Thallium Total | µg/L | 0.8 | |
Uranium Total | µg/L | 10 | |
Vanadium Total | µg/L | 100 | |
Zinc Total | µg/L | 30 | |
Pesticides |
|
| |
Acid Herbicides |
|
| |
2,4-D | µg/L | 4 | |
Bromoxynil | µg/L | 0.33 | |
Dicamba | µg/L | 0.006 | |
MCPA | µg/L | 0.025 | |
Picloram | µg/L | 29 | |
Organochlorine Pesticides in Water |
|
| |
Endosulfan | µg/L | 0.003 | |
Hexachlorocyclohexane (gamma-HCH) (Lindane) | µg/L | 0.01 | |
Hexachlorobenzene | µg/L | 0.52 | |
Pentachlorophenol (PCP) | µg/L | 0.5 | |
Neutral Herbicides in Water |
|
| |
Atrazine | µg/L | 1.8 | |
Diclofopmethyl (Hoegrass) | µg/L | 0.18 | |
Metolachlor | µg/L | 7.8 | |
Metribuzin | µg/L | 0.5 | |
Simazine | µg/L | 0.5 | |
Triallate | µg/L | 0.24 | |
Trifluralin | µg/L | 0.2 | |
Other |
|
| |
Glyphosate | µg/L | Report Detections | |
Fish Tissue |
|
| |
Mercury in fish (muscle tissue) | µg/kg | 200 | |
Arsenic in fish (muscle tissue) | µg/kg | 3500 | |
Lead in fish (muscle tissue) | µg/kg | 500 | |
DDT (total) in fish (muscle tissue) | µg/kg | 5000 | |
Aquatic Biota Consumption |
|
| |
PCB in fish (muscle tissue) mammalian | µg TEQ/kg diet wet weight | 0.00079 | |
PCB in fish (muscle tissue) avian | µg TEQ/kg diet wet weight | 0.0024 | |
DDT (total) in fish (muscle tissue) | µg/kg diet wet weight | 14 | |
Toxaphene in fish (muscle tissue) | µg/kg diet wet weight | 6.3 | |
Radioactive |
|
| |
Cesium-137 | Bq/L | 10 | |
Iodine-131 | Bq/L | 6 | |
Lead-210 | Bq/L | 0.2 | |
Radium-226 | Bq/L | 0.5 | |
Strontium-90 | Bq/L | 5 | |
Tritium | Bq/L | 7000 | |
Red Deer River S/M Reach: Etomami River to Red Deer Lake | |||
Chemical, Physical or Biological Variable | Unit | Acceptable Limit or Limits | |
Nutrients | Open | Closed | |
Total Phosphorus | mg/L | 0.052 | 0.074 |
0.066 | 0.161 | ||
Total Dissolved Phosphorus | mg/L | 0.021 | 0.025 |
0.029 | 0.055 | ||
Total Nitrogen | mg/L | 1.195 | 1.998 |
Nitrate as N | mg/L | 3 | |
Ammonia Un-ionized | mg/L | 0.019a | |
Major Ions |
|
| |
Total Dissolved Solids | mg/L | 500 | |
Sulphate Dissolved | mg/L | 250 | |
Sodium Dissolved | mg/L | 200 | |
Fluoride Dissolved | mg/L | 0.18 | |
Chloride Dissolved | mg/L | 100 | |
Physicals and Other | |||
pH Lab | pH units | 6.5-9.0 | |
pH Field | pH units | 6.5-9.0 | |
Oxygen Dissolved |
|
| |
Temperature > 5°C (Open Season) | mg/L | 5 | |
Temperature < 5°C (Closed Season) | mg/L | 3 | |
Sodium Adsorption Ratio | rel units | 3 | |
Total Suspended Solids | mg/L | 1.0 - 19.7 | |
Reactive Chlorine Species | mg/L | 0.0005 | |
Cyanide (free) | mg/L | 0.005 | |
| |||
E. Coli | No./100 mL | 200 | |
Coliforms Fecal | No./100 mL | 100 | |
Metals | |||
Arsenic Total | µg/L | 5 | |
Arsenic Dissolved | µg/L | No Objective | |
Barium Total | µg/L | 1000 | |
Beryllium Total | µg/L | 100 | |
Boron Total | µg/L | 500 | |
Cadmium Total | µg/L | Calculatedb | |
Chromium Total | µg/L | 50 | |
Cobalt Total | µg/L | 50 | |
Copper Total | µg/L | Calculatedb | |
Iron Dissolved | µg/L | 300 | |
Lead Total | µg/L | Calculatedb | |
Lithium Total | µg/L | 2500 | |
Manganese Dissolved | µg/L | 50 | |
Mercury Total | µg/L | 0.026 | |
Molybdenum Total | µg/L | 10 | |
Nickel Dissolved | µg/L | Calculatedb | |
Selenium Total | µg/L | 1 | |
Silver Total | µg/L | 0.1 | |
Thallium Total | µg/L | 0.8 | |
Uranium Total | µg/L | 10 | |
Vanadium Total | µg/L | 100 | |
Zinc Total | µg/L | 30 | |
Pesticides |
|
|
Acid Herbicides |
|
|
2,4-D | µg/L | 4 |
Bromoxynil | µg/L | 0.33 |
Dicamba | µg/L | 0.006 |
MCPA | µg/L | 0.025 |
Picloram | µg/L | 29 |
Organochlorine Pesticides in Water |
|
|
Endosulfan | µg/L | 0.003 |
Hexachlorocyclohexane (gamma-HCH) (Lindane) | µg/L | 0.01 |
Hexachlorobenzene | µg/L | 0.52 |
Pentachlorophenol (PCP) | µg/L | 0.5 |
Neutral Herbicides in Water |
|
|
Atrazine | µg/L | 1.8 |
Diclofopmethyl (Hoegrass) | µg/L | 0.18 |
Metolachlor | µg/L | 7.8 |
Metribuzin | µg/L | 0.5 |
Simazine | µg/L | 0.5 |
Triallate | µg/L | 0.24 |
Trifluralin | µg/L | 0.2 |
Other |
|
|
Glyphosate | µg/L | Report Detections |
Fish Tissue |
|
|
Mercury in fish (muscle tissue) | µg/kg | 200 |
Arsenic in fish (muscle tissue) | µg/kg | 3500 |
Lead in fish (muscle tissue) | µg/kg | 500 |
DDT (total) in fish (muscle tissue) | µg/kg | 5000 |
Aquatic Biota Consumption |
|
|
PCB in fish (muscle tissue) mammalian | µg TEQ/kg diet wet weight | 0.00079 |
PCB in fish (muscle tissue) avian | µg TEQ/kg diet wet weight | 0.0024 |
DDT (total) in fish (muscle tissue) | µg/kg diet wet weight | 14 |
Toxaphene in fish (muscle tissue) | µg/kg diet wet weight | 6.3 |
Radioactive |
|
|
Cesium-137 | Bq/L | 10 |
Iodine-131 | Bq/L | 6 |
Lead-210 | Bq/L | 0.2 |
Radium-226 | Bq/L | 0.5 |
Strontium-90 | Bq/L | 5 |
Tritium | Bq/L | 7000 |
Red Deer River A/S Reach: Bindloss to Confluence with the S. Sask. River | |||
Chemical, Physical or Biological Variable | Unit | Acceptable Limit or Limits | |
Nutrients | Open | Closed | |
Total Phosphorus | mg/L | 0.315 | 0.035 |
0.563 | 0.069 | ||
Total Dissolved Phosphorus | mg/L | 0.023 | 0.008 |
0.035 | 0.024 | ||
Total Nitrogen | mg/L | 2.320 | 0.860 |
Nitrate as N | mg/L | 3 | |
Ammonia Un-ionized | mg/L | 0.019a | |
Major Ions |
|
| |
Total Dissolved Solids | mg/L | 500 | |
Sulphate Dissolved | mg/L | 250 | |
Sodium Dissolved | mg/L | 200 | |
Fluoride Dissolved | mg/L | 0.2 | |
Chloride Dissolved | mg/L | 100 | |
Physicals and Other | |||
pH Lab | pH units | 6.5-9.0 | |
pH Field | pH units | 6.5-9.0 | |
Oxygen Dissolved |
|
| |
Temperature > 5°C (Open Season) | mg/L | 5 | |
Temperature < 5°C (Closed Season) | mg/L | 3 | |
Sodium Adsorption Ratio | rel units | 3 | |
Total Suspended Solids | mg/L | 30.0-832.6 | |
Reactive Chlorine Species | mg/L | 0.0005 | |
Cyanide (free) | mg/L | 0.005 | |
| |||
E. Coli | No./100 mL | 200 | |
Coliforms Fecal | No./100 mL | 100 | |
Metals | |||
Arsenic Total | µg/L | 5 | |
Arsenic Dissolved | µg/L | No Objective | |
Barium Total | µg/L | 1000 | |
Beryllium Total | µg/L | 100 | |
Boron Total | µg/L | 500 | |
Cadmium Total | µg/L | Under Review | |
Chromium Total | µg/L | 50 | |
Cobalt Total | µg/L | 50 | |
Copper Total | µg/L | Under Review | |
Iron Dissolved | µg/L | 300 | |
Lead Total | µg/L | Calculatedb | |
Lithium Total | µg/L | 2500 | |
Manganese Dissolved | µg/L | 50 | |
Mercury Total | µg/L | 0.026 | |
Molybdenum Total | µg/L | 10 | |
Nickel Dissolved | µg/L | Calculatedb | |
Selenium Total | µg/L | 1 | |
Silver Total | µg/L | 0.1 | |
Thallium Total | µg/L | 0.8 | |
Uranium Total | µg/L | 10 | |
Vanadium Total | µg/L | 100 | |
Zinc Total | µg/L | 30 | |
Pesticides |
|
|
Acid Herbicides |
|
|
2,4-D | µg/L | 4 |
Bromoxynil | µg/L | 0.33 |
Dicamba | µg/L | 0.006 |
MCPA | µg/L | 0.025 |
Picloram | µg/L | 29 |
Organochlorine Pesticides in Water |
|
|
Endosulfan | µg/L | 0.003 |
Hexachlorocyclohexane (gamma-HCH) (Lindane) | µg/L | 0.01 |
Hexachlorobenzene | µg/L | 0.52 |
Pentachlorophenol (PCP) | µg/L | 0.5 |
Neutral Herbicides in Water |
|
|
Atrazine | µg/L | 1.8 |
Diclofopmethyl (Hoegrass) | µg/L | 0.18 |
Metolachlor | µg/L | 7.8 |
Metribuzin | µg/L | 0.5 |
Simazine | µg/L | 0.5 |
Triallate | µg/L | 0.24 |
Trifluralin | µg/L | 0.2 |
Other |
|
|
Glyphosate | µg/L | Report Detections |
Fish Tissue |
|
|
Mercury in fish (muscle tissue) | µg/kg | 200 |
Arsenic in fish (muscle tissue) | µg/kg | 3500 |
Lead in fish (muscle tissue) | µg/kg | 500 |
DDT (total) in fish (muscle tissue) | µg/kg | 5000 |
Aquatic Biota Consumption |
|
|
PCB in fish (muscle tissue) mammalian | µg TEQ/kg diet wet weight | 0.00079 |
PCB in fish (muscle tissue) avian | µg TEQ/kg diet wet weight | 0.0024 |
DDT (total) in fish (muscle tissue) | µg/kg diet wet weight | 14 |
Toxaphene in fish (muscle tissue) | µg/kg diet wet weight | 6.3 |
Radioactive |
|
|
Cesium-137 | Bq/L | 10 |
Iodine-131 | Bq/L | 6 |
Lead-210 | Bq/L | 0.2 |
Radium-226 | Bq/L | 0.5 |
Strontium-90 | Bq/L | 5 |
Tritium | Bq/L | 7000 |
Saskatchewan River Reach: Outlet of Cumberland Lake to Mouth of Carrot River | |||
Chemical, Physical or Biological Variable | Unit | Acceptable Limit or Limits | |
Nutrients | Open | Closed | |
Total Phosphorus | mg/L | 0.088 | 0.028 |
0.124 | 0.034 | ||
Total Dissolved Phosphorus | mg/L | 0.014 | 0.011 |
0.018 | 0.017 | ||
Total Nitrogen | mg/L | 0.838 | 0.761 |
Nitrate as N | mg/L | 3 | |
Ammonia Un-ionized | mg/L | 0.019a | |
Major Ions |
|
| |
Total Dissolved Solids | mg/L | 500 | |
Sulphate Dissolved | mg/L | 250 | |
Sodium Dissolved | mg/L | 200 | |
Fluoride Dissolved | mg/L | 0.18 | |
Chloride Dissolved | mg/L | 100 | |
Physicals and Other | |||
pH Lab | pH units | 6.5-9.0 | |
pH Field | pH units | 6.5-9.0 | |
Oxygen Dissolved |
|
| |
Temperature > 5°C (Open Season) | mg/L | 5 | |
Temperature < 5°C (Closed Season) | mg/L | 3 | |
Sodium Adsorption Ratio | rel units | 3 | |
Total Suspended Solids | mg/L | 27.0 - 125.0 | |
Reactive Chlorine Species | mg/L | 0.0005 | |
Cyanide (free) | mg/L | 0.005 | |
| |||
E. Coli | No./100 mL | 200 | |
Coliforms Fecal | No./100 mL | 100 | |
Metals | |||
Arsenic Total | µg/L | 5 | |
Arsenic Dissolved | µg/L | No Objective | |
Barium Total | µg/L | 1000 | |
Beryllium Total | µg/L | 100 | |
Boron Total | µg/L | 500 | |
Cadmium Total | µg/L | Calculatedb | |
Chromium Total | µg/L | 50 | |
Cobalt Total | µg/L | 50 | |
Copper Total | µg/L | Calculatedb | |
Iron Dissolved | µg/L | 300 | |
Lead Total | µg/L | Calculatedb | |
Lithium Total | µg/L | 2500 | |
Manganese Dissolved | µg/L | 50 | |
Mercury Total | µg/L | 0.026 | |
Molybdenum Total | µg/L | 10 | |
Nickel Dissolved | µg/L | Calculatedb | |
Selenium Total | µg/L | 1 | |
Silver Total | µg/L | 0.1 | |
Thallium Total | µg/L | 0.8 | |
Uranium Total | µg/L | 10 | |
Vanadium Total | µg/L | 100 | |
Zinc Total | µg/L | 30 | |
Pesticides |
|
|
Acid Herbicides |
|
|
2,4-D | µg/L | 4 |
Bromoxynil | µg/L | 0.33 |
Dicamba | µg/L | 0.006 |
MCPA | µg/L | 0.025 |
Picloram | µg/L | 29 |
Organochlorine Pesticides in Water |
|
|
Endosulfan | µg/L | 0.003 |
Hexachlorocyclohexane (gamma-HCH) (Lindane) | µg/L | 0.01 |
Hexachlorobenzene | µg/L | 0.52 |
Pentachlorophenol (PCP) | µg/L | 0.5 |
Neutral Herbicides in Water |
|
|
Atrazine | µg/L | 1.8 |
Diclofopmethyl (Hoegrass) | µg/L | 0.18 |
Metolachlor | µg/L | 7.8 |
Metribuzin | µg/L | 0.5 |
Simazine | µg/L | 0.5 |
Triallate | µg/L | 0.24 |
Trifluralin | µg/L | 0.2 |
Other |
|
|
Glyphosate | µg/L | Report Detections |
Fish Tissue |
|
|
Mercury in fish (muscle tissue) | µg/kg | 200 |
Arsenic in fish (muscle tissue) | µg/kg | 3500 |
Lead in fish (muscle tissue) | µg/kg | 500 |
DDT (total) in fish (muscle tissue) | µg/kg | 5000 |
Aquatic Biota Consumption |
|
|
PCB in fish (muscle tissue) mammalian | µg TEQ/kg diet wet weight | 0.00079 |
PCB in fish (muscle tissue) avian | µg TEQ/kg diet wet weight | 0.0024 |
DDT (total) in fish (muscle tissue) | µg/kg diet wet weight | 14 |
Toxaphene in fish (muscle tissue) | µg/kg diet wet weight | 6.3 |
Radioactive |
|
|
Cesium-137 | Bq/L | 10 |
Iodine-131 | Bq/L | 6 |
Lead-210 | Bq/L | 0.2 |
Radium-226 | Bq/L | 0.5 |
Strontium-90 | Bq/L | 5 |
Tritium | Bq/L | 7000 |
South Sask. River Reach: Highway #41 to Confluence with Red Deer River | |||
Chemical, Physical or Biological Variable | Unit | Acceptable Limit or Limits | |
Nutrients | Open | Closed | |
Total Phosphorus | mg/L | 0.159 | 0.054 |
0.246 | 0.110 | ||
Total Dissolved Phosphorus | mg/L | 0.014 | 0.010 |
0.018 | 0.067 | ||
Total Nitrogen | mg/L | 1.073 | 1.638 |
1.114 | 1.771 | ||
Nitrate as N | mg/L | 3 | |
Ammonia Un-ionized | mg/L | 0.019a | |
Major Ions |
|
| |
Total Dissolved Solids | mg/L | 500 | |
Sulphate Dissolved | mg/L | 250 | |
Sodium Dissolved | mg/L | 200 | |
Fluoride Dissolved | mg/L | 0.19 | |
Chloride Dissolved | mg/L | 100 | |
Physicals and Other | |||
pH Lab | pH units | 6.5-9.0 | |
pH Field | pH units | 6.5-9.0 | |
Oxygen Dissolved |
|
| |
Temperature > 5°C (Open Season) | mg/L | 5 | |
Temperature < 5°C (Closed Season) | mg/L | 3 | |
Sodium Adsorption Ratio | rel units | 3 | |
Total Suspended Solids | mg/L | 5.6-339.8 | |
Reactive Chlorine Species | mg/L | 0.0005 | |
Cyanide (free) | mg/L | 0.005 | |
| |||
E. Coli | No./100 mL | 200 | |
Coliforms Fecal | No./100 mL | 100 | |
Metals | |||
Arsenic Total | µg/L | 5 | |
Arsenic Dissolved | µg/L | No Objective | |
Barium Total | µg/L | 1000 | |
Beryllium Total | µg/L | 100 | |
Boron Total | µg/L | 500 | |
Cadmium Total | µg/L | Calculatedb | |
Chromium Total | µg/L | 50 | |
Cobalt Total | µg/L | 50 | |
Copper Total | µg/L | Calculatedb | |
Iron Dissolved | µg/L | 300 | |
Lead Total | µg/L | Calculatedb | |
Lithium Total | µg/L | 2500 | |
Manganese Dissolved | µg/L | 50 | |
Mercury Total | µg/L | 0.026 | |
Molybdenum Total | µg/L | 10 | |
Nickel Dissolved | µg/L | Calculatedb | |
Selenium Total | µg/L | 1 | |
Silver Total | µg/L | 0.1 | |
Thallium Total | µg/L | 0.8 | |
Uranium Total | µg/L | 10 | |
Vanadium Total | µg/L | 100 | |
Zinc Total | µg/L | 30 | |
Pesticides |
|
|
Acid Herbicides |
|
|
2,4-D | µg/L | 4 |
Bromoxynil | µg/L | 0.33 |
Dicamba | µg/L | 0.006 |
MCPA | µg/L | 0.025 |
Picloram | µg/L | 29 |
Organochlorine Pesticides in Water |
|
|
Endosulfan | µg/L | 0.003 |
Hexachlorocyclohexane (gamma-HCH) (Lindane) | µg/L | 0.01 |
Hexachlorobenzene | µg/L | 0.52 |
Pentachlorophenol (PCP) | µg/L | 0.5 |
Neutral Herbicides in Water |
|
|
Atrazine | µg/L | 1.8 |
Diclofopmethyl (Hoegrass) | µg/L | 0.18 |
Metolachlor | µg/L | 7.8 |
Metribuzin | µg/L | 0.5 |
Simazine | µg/L | 0.5 |
Triallate | µg/L | 0.24 |
Trifluralin | µg/L | 0.2 |
Other |
|
|
Glyphosate | µg/L | Report Detections |
Fish Tissue |
|
|
Mercury in fish (muscle tissue) | µg/kg | 200 |
Arsenic in fish (muscle tissue) | µg/kg | 3500 |
Lead in fish (muscle tissue) | µg/kg | 500 |
DDT (total) in fish (muscle tissue) | µg/kg | 5000 |
Aquatic Biota Consumption |
|
|
PCB in fish (muscle tissue) mammalian | µg TEQ/kg diet wet weight | 0.00079 |
PCB in fish (muscle tissue) avian | µg TEQ/kg diet wet weight | 0.0024 |
DDT (total) in fish (muscle tissue) | µg/kg diet wet weight | 14 |
Toxaphene in fish (muscle tissue) | µg/kg diet wet weight | 6.3 |
Radioactive |
|
|
Cesium-137 | Bq/L | 10 |
Iodine-131 | Bq/L | 6 |
Lead-210 | Bq/L | 0.2 |
Radium-226 | Bq/L | 0.5 |
Strontium-90 | Bq/L | 5 |
Tritium | Bq/L | 7000 |
Related Files
The MAA provides for an equitable sharing of available waters for all eastward flowing streams that cross interprovincial boundaries, including lakes and serves to protect transboundary aquifers and surface water quality.
